Greg Landrum
Greg Landrum
Hi @wopozka, this is also what the IUPAC recommends (https://www.degruyter.com/document/doi/10.1351/pac200678101897/html?lang=en): 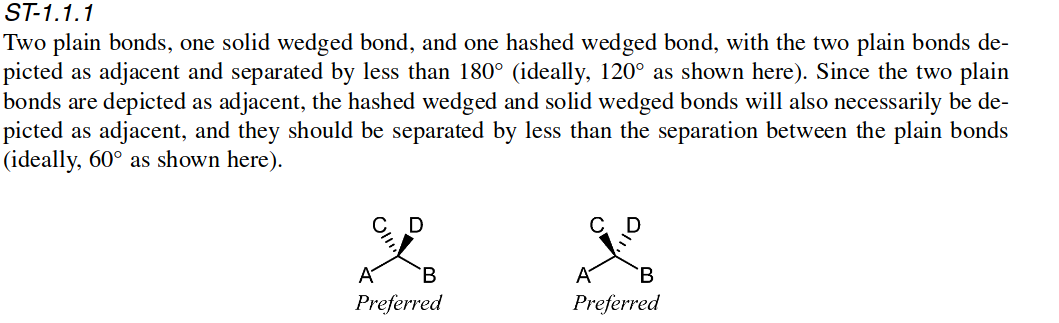 and I agree that it can be clearer. We need to revisit the bond wedging code anyway as part...
@wopozka : I'm not sure what your use case is, but what the RDKit current does is generally unambiguous and is a quite common way of depicting atomic sterochemistry (IUPAC...
Hi @zacps : this is a known shortcoming in the RDKit. The toolkit does not support that kind of query
@philopon : thanks for the fixing this oversight in the minimal lib wrappers!
@philopon : would it help if I write a test for the new functionality and add it to your PR?
Adding on to @bp-kelley's answer: it's als possible to specify the ring closures with 1-5 digits by putting them inside parentheses (this is a SMILES extension): `C%(1)CC%(1)` This is documented...
I've looked at a few of these structures and what they have in common is a fused ring system with an impossible stereochemistry arrangement. Here's the first molecule from that...
@cdvonbargen I think the idea of having names available is a good one, but adding them to directly to the periodic table seems not-quite-right. Between the possibility of alternate spellings...
Superseded by #5524